Draw the major elimination product formed in the reaction. – Draw the major elimination product formed in the reaction sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This comprehensive guide delves into the intricacies of elimination reactions, providing a thorough examination of their mechanisms, regioselectivity, stereochemistry, and applications.
Delving into the heart of the topic, we will explore the fundamental concepts of elimination reactions, shedding light on the E1 and E2 mechanisms. Through illustrative examples, we will unravel the factors that govern the regioselectivity of these reactions, empowering you to predict the major elimination product based on the structure of the starting material.
Elimination Reaction Mechanisms
Elimination reactions are chemical reactions that involve the removal of two substituents from adjacent carbon atoms, resulting in the formation of a double bond or a triple bond. There are two main types of elimination mechanisms: E1 and E2.
E1 Mechanism
In the E1 mechanism, the first step is the ionization of the substrate to form a carbocation. This is followed by the removal of a proton from a carbon adjacent to the carbocation by a base. The E1 mechanism is favored by polar solvents, which stabilize the carbocation intermediate, and by tertiary substrates, which form more stable carbocations.
E2 Mechanism, Draw the major elimination product formed in the reaction.
In the E2 mechanism, the proton and the leaving group are removed in a concerted manner. This means that the breaking of the C-H bond and the C-X bond occurs simultaneously. The E2 mechanism is favored by nonpolar solvents, which do not stabilize the carbocation intermediate, and by primary substrates, which form less stable carbocations.
Regioselectivity of Elimination Reactions
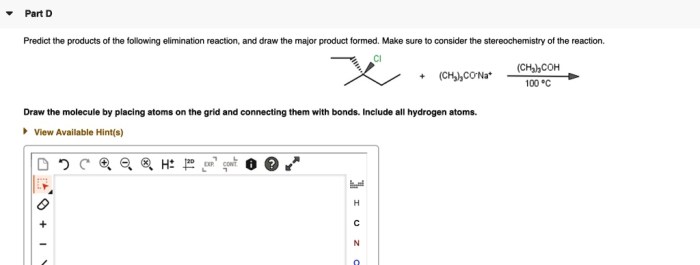
Regioselectivity refers to the preference for one product over another in a reaction. In elimination reactions, the regioselectivity is determined by the stability of the double bond or triple bond that is formed.
Zaitsev’s Rule
Zaitsev’s rule states that the more substituted alkene or alkyne is the major product of an elimination reaction. This is because the more substituted double bond or triple bond is more stable.
Hofmann’s Rule
Hofmann’s rule states that the less substituted alkene or alkyne is the major product of an elimination reaction when the base is bulky. This is because the bulky base is more likely to abstract a proton from a less substituted carbon atom.
Stereochemistry of Elimination Reactions: Draw The Major Elimination Product Formed In The Reaction.
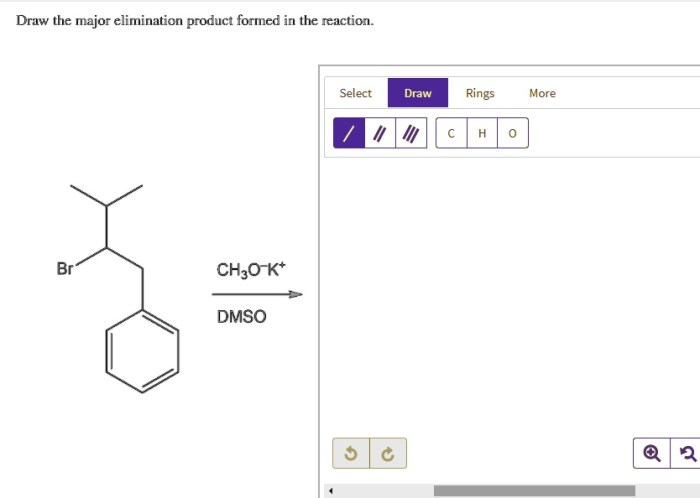
The stereochemistry of elimination reactions is determined by the geometry of the starting material and the reaction mechanism.
E2 Reactions
E2 reactions occur via a concerted mechanism, which means that the proton and the leaving group are removed in a synchronous manner. This results in the formation of an alkene or alkyne with a trans configuration.
Anti-Periplanar Geometry
The anti-periplanar geometry is the preferred geometry for E2 reactions. In this geometry, the proton and the leaving group are located on opposite sides of the double bond and are in the same plane.
Applications of Elimination Reactions
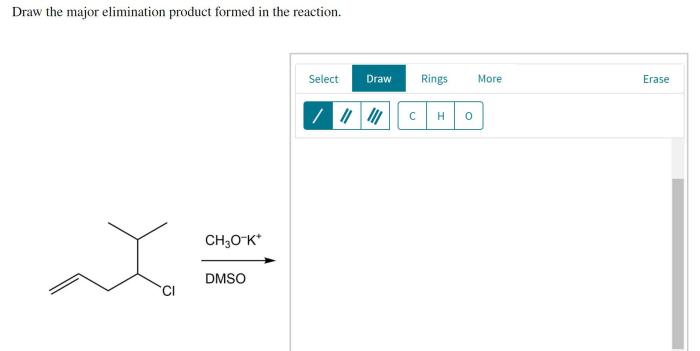
Elimination reactions are used in a variety of organic synthesis applications.
Formation of Alkenes and Alkynes
Elimination reactions are commonly used to form alkenes and alkynes. This is done by reacting an alkyl halide with a strong base.
Synthesis of Natural Products
Elimination reactions are also used in the synthesis of natural products. For example, the elimination of water from a hydroxy ketone is used to form an enone, which is a common structural motif in many natural products.
FAQ Section
What is the E2 mechanism?
The E2 mechanism is a concerted elimination reaction that occurs in a single step. The base abstracts a proton from the carbon adjacent to the leaving group, and the leaving group departs simultaneously, resulting in the formation of an alkene.
What is regioselectivity?
Regioselectivity refers to the preference for one product over another in a reaction. In elimination reactions, regioselectivity is determined by the stability of the alkene product. The more substituted alkene is generally the more stable and therefore the major product.
What is stereochemistry?
Stereochemistry refers to the spatial arrangement of atoms in a molecule. In elimination reactions, stereochemistry is determined by the geometry of the starting material and the reaction mechanism. The E2 mechanism typically results in the formation of an anti-periplanar alkene.